Equity X-Ray: In-Depth Research #14
🧪 Revolutionizing Healthcare with CRISPR-Based Breakthroughs🧬
Company Overview
Intellia Therapeutics is a pioneering biotechnology company at the forefront of gene editing, leveraging CRISPR-based technologies to develop transformative therapies. With a mission to address significant unmet medical needs, Intellia is committed to delivering single-dose, potentially curative treatments for severe genetic diseases. The company’s innovative approach combines cutting-edge science with a patient-centric focus, aiming to revolutionize the treatment landscape for conditions like hereditary angioedema (HAE) and transthyretin amyloidosis (ATTR).
Intellia’s success is driven by its ability to integrate advanced CRISPR technology with deep clinical expertise, resulting in breakthrough therapies that target the root cause of diseases.
🌱 Support Our Work: Buy Us a Coffee or Shop Our Services! 🌱
Your small gesture fuels our big dreams. Click below to make a difference today.
[☕ Buy Us a Coffee]
[🛒 Visit Our Shop]
Business Model
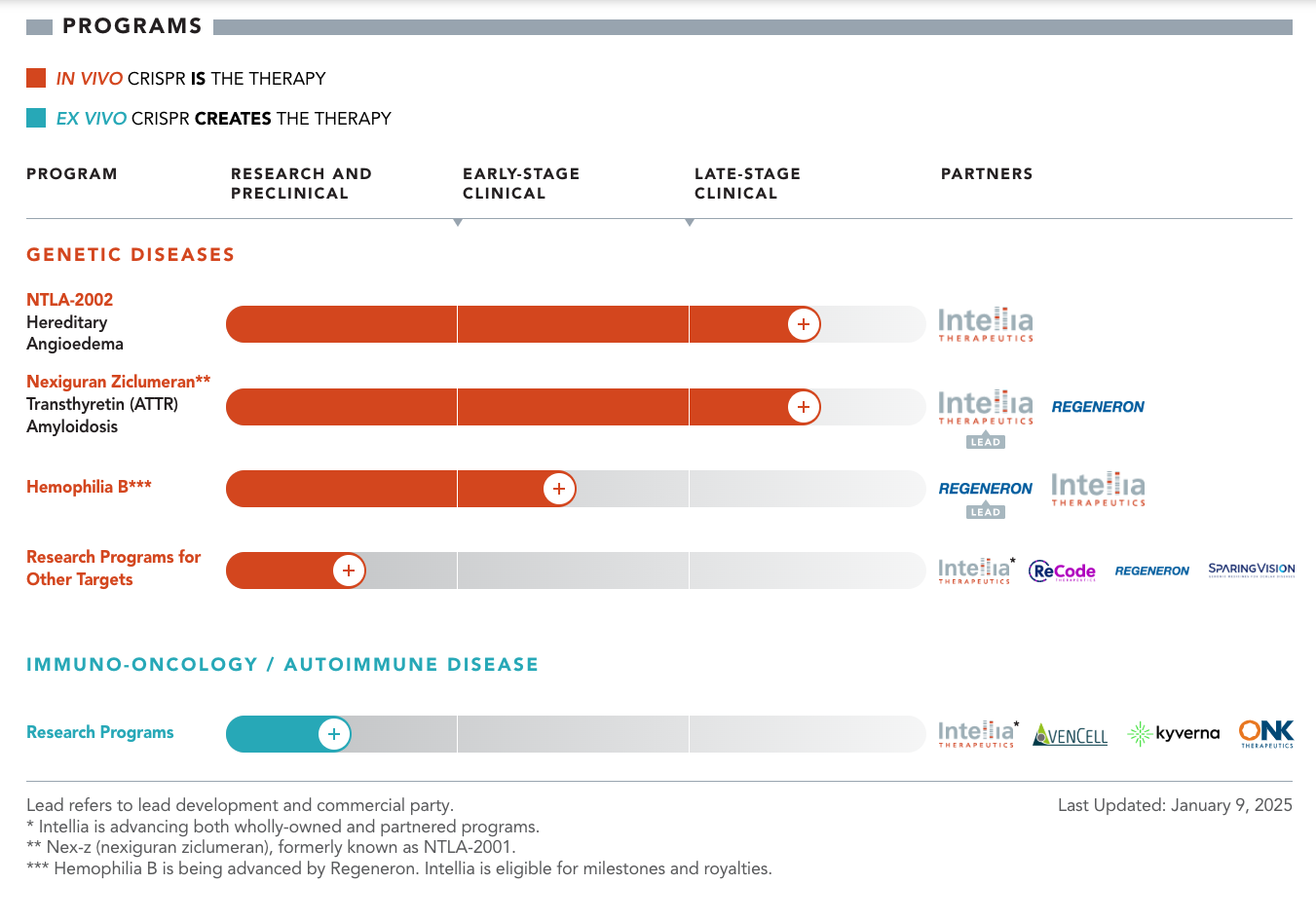
The company's primary focus is developing both in vivo and ex vivo CRISPR-based therapies for genetic diseases. Their lead clinical programs include NTLA-2002 for hereditary angioedema (HAE) and nexiguran ziclumeran (nex-z, formerly NTLA-2001) for transthyretin (ATTR) amyloidosis. These programs represent the cornerstone of Intellia's clinical pipeline and demonstrate the company's commitment to addressing serious genetic conditions with high unmet medical needs.
Intellia's current revenue primarily derives from collaboration agreements with pharmaceutical partners. The company has established strategic partnerships to leverage external expertise while maintaining control of key assets. This collaborative approach allows Intellia to access additional funding and expertise while continuing to advance its proprietary pipeline. The most notable collaboration appears to be with Regeneron for the development of nex-z for ATTR amyloidosis.
Core Business Segments
The company is currently conducting three pivotal Phase 3 trials targeting Hereditary Angioedema (HAE) and Transthyretin Amyloidosis (ATTR). These trials aim to validate therapies that could redefine treatment standards, offering single-dose, potentially curative solutions. Let’s explore these trials and the diseases they address in detail.
Hereditary Angioedema (HAE) and NTLA-2002
Understanding HAE
Hereditary Angioedema is a rare and life-threatening genetic disorder that causes sudden, severe swelling in various parts of the body, including the skin, gastrointestinal tract, and airways. Imagine waking up one day unable to breathe because your throat has swollen shut—this is the terrifying reality for many HAE patients. These attacks are unpredictable and can be triggered by stress, trauma, or even hormonal changes.
The root cause of HAE lies in a mutation in the SERPING1 gene, which disrupts the production of a protein called C1-inhibitor. This leads to an overproduction of bradykinin, a molecule that causes blood vessels to leak fluid, resulting in swelling. Current treatments, while effective to some extent, require frequent injections or pills, leaving patients with a significant treatment burden and ongoing anxiety about their next attack.
The Promise of NTLA-2002
NTLA-2002 is a CRISPR-based therapy designed to address the root cause of HAE. By targeting the KLKB1 gene in liver cells, it reduces the production of kallikrein, an enzyme that drives bradykinin production. In simpler terms, it’s like turning off the faucet that causes the swelling.
The Phase 3 trial for NTLA-2002 involves a single intravenous infusion, to significantly reduce or even eliminate swelling attacks. Early results are promising: in Phase 2, 73% of patients were completely attack-free after just one dose, and the effects lasted for up to two years. The safety profile has also been encouraging, with only mild side effects like headaches and fatigue reported.
If successful, NTLA-2002 could offer patients lifelong freedom from attacks and eliminate the need for chronic treatments, transforming their quality of life.
Transthyretin Amyloidosis (ATTR) and Nexiguran Ziclumeran (Nex-z)
What is ATTR?
Transthyretin Amyloidosis is a progressive and often fatal disease caused by the buildup of misfolded transthyretin (TTR) protein in the body. This protein can deposit in various tissues, leading to two major forms of the disease: ATTR with Cardiomyopathy (ATTR-CM) and ATTR with Polyneuropathy (ATTRv-PN).
ATTR-CM primarily affects the heart, causing it to stiffen and lose its ability to pump blood effectively. Patients experience symptoms like shortness of breath, fatigue, and arrhythmias, often leading to heart failure.
ATTRv-PN, on the other hand, affects the nerves, leading to numbness, pain, muscle weakness, and severe gastrointestinal issues. Over time, it can rob patients of their mobility and independence.
Current treatments for ATTR slow the disease but do not reverse it. They also require frequent dosing, which can be burdensome for patients.
Nex-z: A Game-Changer for ATTR
Nexiguran Ziclumeran (Nex-z) is a CRISPR-based therapy that targets the TTR gene in liver cells, halting the production of misfolded TTR protein. This approach not only stops the disease from progressing but also has the potential to reverse some of the damage.
1. Nex-z for ATTR-CM
The Phase 3 trial for ATTR-CM is one of the largest of its kind, enrolling approximately 765 patients. Participants receive a single intravenous infusion of Nex-z, and the trial will measure outcomes like cardiovascular-related mortality, heart function, and quality of life. Early data from Phase 1 showed an 89% reduction in TTR levels after a single dose, with many patients experiencing stabilization or improvement in heart function.
For patients, this could mean fewer hospitalizations, improved mobility, and a longer, healthier life. Imagine going from struggling to climb a flight of stairs to being able to enjoy a walk in the park again—that’s the kind of impact Nex-z could have.
2. Nex-z for ATTRv-PN
The trial for ATTRv-PN focuses on patients with nerve damage caused by TTR deposits. Participants receive the same single-dose treatment, and the trial measures improvements in neuropathy symptoms, quality of life, and TTR levels. Phase 1 results showed a 90% reduction in TTR levels, with many patients reporting significant relief from symptoms like pain and numbness.
For these patients, Nex-z offers hope of regaining lost function and independence. It’s not just about stopping the disease—it’s about giving people their lives back.
Market Analysis
Total Addressable Market (TAM)
Definition: The global revenue opportunity for CRISPR-based gene editing therapies across all genetic diseases is addressable by Intellia’s platform.
Key Components:
Monogenic Diseases:
CRISPR’s addressable market for monogenic diseases exceeds $75 billion annually, covering over 7,000 rare genetic disorders with high unmet needs.
Includes lead indications such as ATTR amyloidosis, hereditary angioedema (HAE), and cystic fibrosis.